Electrode protection in acid environment
Electrode protection in acid environment
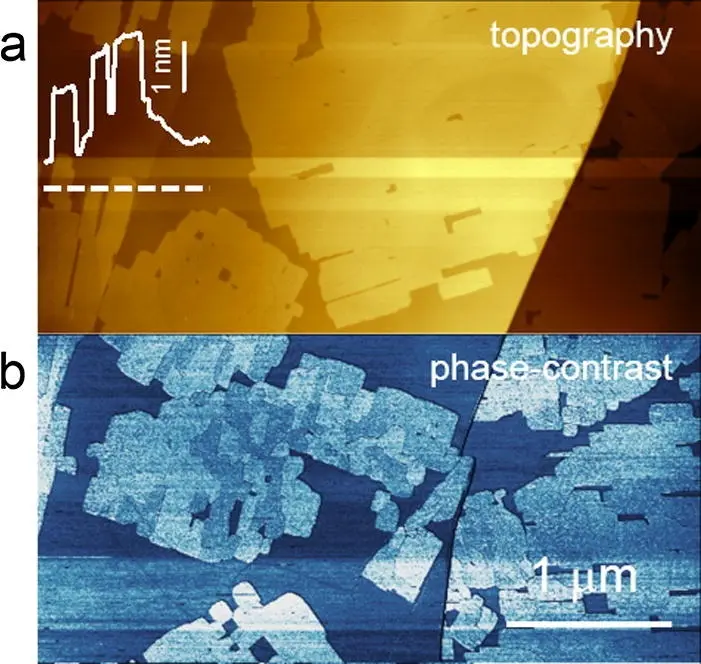
The proposed thesis aims to study the effect of some molecules (namely porphryins) in the protection of graphite electrodes when immersed inside acid solutions (typically sulfuric acid). In particular, graphite electrodes are used in modern batteries and they undergo a significant detriment due to the acid solution. Consequently, new protection strategies (easy preparation procedure, low costs) are under investigation. Recently, we observed that some organic compounds, such as porphyrins, can protect the electrode surface from detriment. The physical-chemical rationale is still under debate. An in-depth study of this topic requires the use of an electrochemical cell in which the potential applied on the sample can be tuned in view of driving different chemical reactions. On the other hand, the study of the organic film stability on the graphite surface at the atomic/molecular scale requires the use of a high-resolution microscopy like AFM (Atomic Force Microscopy) and/or STM (scanning tunneling microscopy). These experimental systems generally work on samples kept in air or in a vacuum environment. An advanced set-up allows to plunge the head of the microscope directly inside the solution [electrochemical AFM / STM (EC-AFM / STM)]. In the figure below, an image of the HOPG electrode covered by porphyrin crystals is acquired with the EC-AFM. We require that the undergraduate student has a basic knowledge of chemistry. This is a prerequisite in view of handling simple chemical preparations used during the experiments. After a quick training on the use of the EC-AFM / STM, the undergraduate student will be involved in the acquisition of EC-AFM/STM images, data analysis and correlation with other specific chemical analysis [i.e. cyclic voltammetry (CV)]. The thesis is a full-time (from Monday to Friday) work that takes at least nine months.
References.
M. Penconi et al., "Customised porphyrin coating films for graphite electrode protection: An investigation on the role of peripheral groups by coupled AFM and cyclic voltammetry techniques", Appl. Surf. Sci. 507 (2020) 145055.